Abstract
Background
Although previous studies demonstrated the presence and prognostic significance of clonal circulating plasma cells (cPCs) detected by multiparameter flow cytometry (MFC) in patients with multiple myeloma (MM), monocle gammopathy of undetermined significance (MGUS), and smoldering MM (sMM), most were conducted during the pre-novel agent era. The relationship between cPC quantification and the revised international staging system (R-ISS) has not been elucidated thus far. We retrospectively analyzed the prognostic significance of cPCs in patients with newly diagnosed MM (NDMM) receiving novel agent-containing induction therapies and their relationship with R-ISS.
Patients and methods
From November 2007 to April 2017, we evaluated clonal cPCs of newly diagnosed, symptomatic MM patients admitted to Kameda Medical Center, Kamogowa-shi, Japan. We retrospectively analyzed clonal cPCs at diagnosis. Diagnosis and assessment of response to treatment were made according to IMWG criteria. Survival analysis was performed using Kaplan-Meier curve and differences were assessed by log-rank test. Two-tube 6- or 7-color MFC was performed on peripheral blood mononuclear cells (MNCs), isolated by the Ficoll-Hypaque density gradient]. We stained MNCs with monoclonal antibodies to CD45, CD38, CD19, CD138, CD56, and cytoplasmic kappa and lambda light chains. To achieve a sensitivity of 0.01%, we required 500,000 events. Negative cPC was defined as <1 clonal cPC detected by MFC among 10,000 events. Clonal cPCs were expressed as the number per 500,000 total events. Receiver operating characteristics (ROC) analysis determined the optimal cutoff levels of cPCs for predicting the highest risk of disease progression within 3 years for all patients and patients with R-ISS stage Ⅱ, and disease progression within 2 years for patients with R-ISS stage Ⅲ.
Results
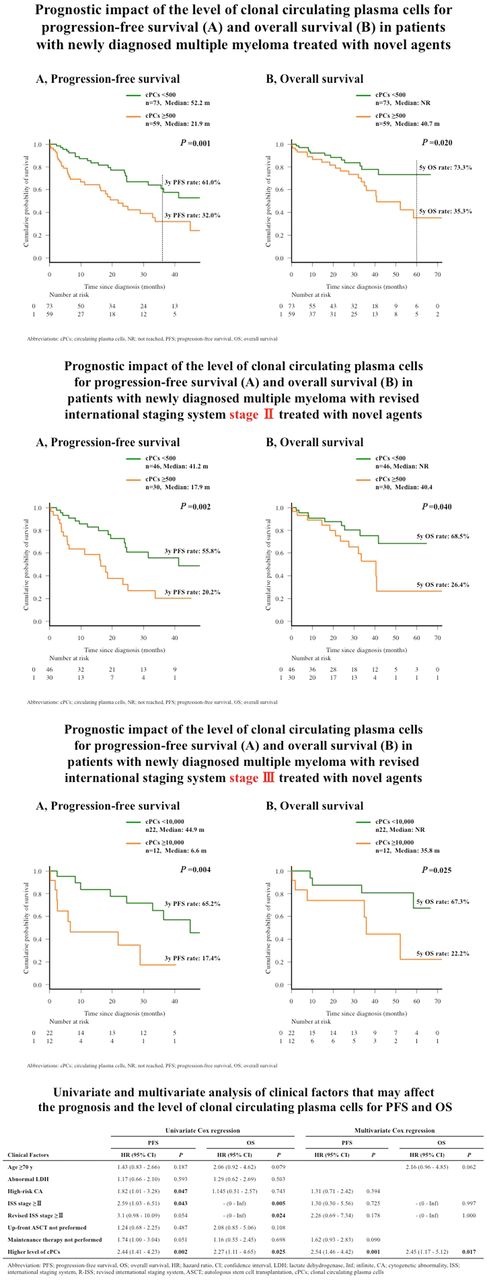
Clonal cPCs were measured in 132 patients and detected in 95 patients (72.0%). The median number of clonal cPCs was 228 [interquartile range (IQR), 28-1,362] per 500,000 total MNCs. ROC analysis showed the optimal cutoff for predicting the highest risk for disease progression within 3 years, in all cohorts, to be 500 cPCs per 500,000 MNCs, yielding an area under the curve (AUC) of 0.649 with a sensitivity of 65.0% and a specificity of 64.1%. All 132 patients were divided into patients with higher (≥500) and lower (<500) cPCs. Comparing the higher and lower clonal cPCs groups, male sex, patients with advanced clinical stage (ISS, R-ISS, and Durie-Salmon stage), albumin, β2-microglobulin, hemoglobin, lactate dehydrogenase, and presence of high-risk cytogenetic abnormality (CA) were significantly associated with the higher clonal cPC group. Survival analysis showed that patients in the higher clonal cPC group had significantly shorter progression-free survival (PFS) [median survival; 52.2 vs 21.9 months (m), P=0.001] and overall survival (OS; median survival; not reached vs 40.7 m, P=0.02). These survival differences remained significant when separately analyzed in patients with R-ISS stage II and stage III (median PFS, 17.9 vs. 41.2 m; P=0.002, and median OS, 40.4 m vs. not reached; P=0.040, in patients with R-ISS stage II, and median PFS, 6.6 vs. 44.9 m; P=0.004, and median OS, 35.8 m vs. not reached; P=0.025, in patients with R-ISS stage III). We used univariate analysis to assess clinical variables and determine their survival effects. High-risk CA, ISS ≥stage II, and higher cPCs were significantly associated with a shorter PFS and ISS. For patients with R-ISS ≥stage II, higher cPCs were associated with OS. Multivariate analysis showed higher cPCs to be independent predictors of poor PFS after adjusting for high-risk CA, ISS ≥stage II, R-ISS ≥stage II and maintenance therapy (P=0.001).cPCs also independently predicted poor OS after adjusting for age, ISS ≥stage II, and R-ISS ≥stage II (P=0.017).
Conclusion
Quantification of cPCs by MFC predicts PFS and OS in patients with NDMM, treated with novel agents. It also showed prognostic significance in patients with the same R-ISS categories (both stage Ⅱ and Ⅲ,), suggesting that it is an independent prognostic predictor of R-ISS and has potential for refining the current prognostic system, including R-ISS.
Kitadate: Toyama kagaku: Research Funding; Otsuka: Research Funding; Eisai: Research Funding; Kyowa Kirin: Research Funding; Fujimoto: Research Funding; Pfizer: Research Funding; Novartis: Research Funding; Asahi Kasei: Research Funding; Chugai: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal